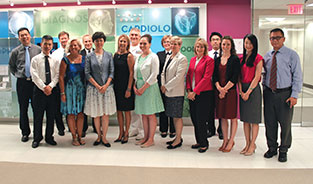
ASTM Staff Meets with China Medical Device Delegation
ASTM staff members Teresa Cendrowska, sixth from right; and Kimberly Simms, fifth from right, were among those meeting with a delegation from the China Medical Device Standardization Administration.
On June 20, ASTM staff members Teresa Cendrowska, vice president, global cooperation, and Kimberly Simms, manager, global cooperation, participated in a meeting with a delegation from the China Medical Device Standardization Administration. In addition to Cendrowska and Simms, the meeting, hosted by the American National Standards Institute, included representatives of the U.S. Food and Drug Administration as well as other U.S.-based organizations that develop standards for medical devices. The meeting was held at the offices of AdvaMed, a medical technology trade association in Washington, D.C.
Since China is considering changes to its regulatory system for medical devices, the meeting offered the opportunity to engage the Chinese visitors in discussions about how the U.S. private sector develops international standards for the medical device industry and the U.S. FDA's use of these standards for regulation and recognition.
Cendrowska and Simms were joined by Jong Yoon Jun, assistant director, Korean Agency for Technology and Standards, who is currently spending 11 months as an attachment at ASTM International headquarters.
 SN Home
SN Home Archive
Archive Advertisers
Advertisers Masthead
Masthead RateCard
RateCard Subscribe
Subscribe Email Editor
Email Editor