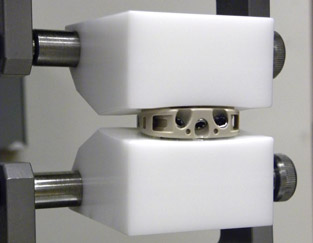
Intervertebral Body Fusion
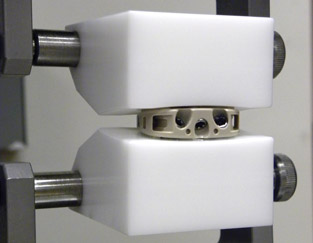
Prototype intervertebral body fusion device being tested per WK34549.
New functionalities in a particular group of orthopedic spinal devices will be addressed in a proposed new ASTM standard. WK34549, Test Methods for Intervertebral Body Fusion Devices with Integrated Fixation Components, is being developed by Subcommittee F04.25 on Spinal Devices, part of ASTM International Committee F04 on Medical and Surgical Materials and Devices.
According to Andrew Dooris, Ph.D, principal engineer, DePuy Spine, and the F04 member leading the WK34549 task group, intervertebral fusion devices have been in use for approximately 15 years. These devices stabilize the spine after discectomy surgery, and act as spacers to keep the vertebral bodies separated in order to prevent nerve compression. The devices are usually supplemented by additional fixation, such as pedicle screw hardware.
"In the past few years there has been a proliferation of devices similar to these intervertebral body fusion devices, in that they too are spacers made of similar materials in similar shapes and inserted with similar techniques, but with an added functionality - they have screws, blades or hooks that latch onto or dig into the adjacent bone," says Dooris. "These additional components may serve to prevent implant migration or expulsion or may help prevent separation of the bone from the device when under significant body torque. In some cases, these devices may obviate the need for additional hardware."
Dooris says that WK34549 is being written primarily to address the added functionalities of these new devices.
"The proposed test method will be useful to manufacturers and the U.S. Food and Drug Administration by providing additional guidance on how to evaluate this new breed of intervertebral body fusion devices and perhaps even guide future designs," says Dooris. Once approved, the proposed standard will used by medical device manufacturers, the U.S. FDA, international regulatory bodies and contract test laboratories. All interested parties are encouraged to join in the standards developing activities of F04.
CONTACT Technical Information: Andrew Dooris, Ph.D., DePuy Spine • Raynham, Mass. • Phone: 508-828-3433 O ASTM Staff: Pat Picariello • Phone: 610-832-9720 O Upcoming Meeting: May 8-11 • May Committee Week • Phoenix, Ariz.
 SN Home
SN Home Archive
Archive Advertisers
Advertisers Masthead
Masthead RateCard
RateCard Subscribe
Subscribe Email Editor
Email Editor