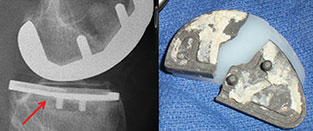
Unicondylar Knee Joint Replacements
A proposed new ASTM International standard will cover fatigue testing of metallic tibial trays, which are components used in partial knee joint replacement. WK45235, Practice for Cyclic Fatigue Testing of Metal Tibial Tray Components of Unicondylar Knee Joint Replacements, is being developed by Subcommittee F04.22 on Arthroplasty, part of ASTM International Committee F04 on Medical and Surgical Materials and Devices.
According to Gokce Yildirim, research engineer, Stryker MAKO, and a member of F04, the test in WK45235 is for materials used in transplants for a middle-aged population (40 and up) who have arthritis forming on the medial side of their knees, but who have healthy cruciate ligaments. "Solving the arthritis pain issue is known to restore normal knee function and give patients more mobility for their daily lives," says Yildirim.
Yildirim notes that the testing described in WK45235 mirrors fatigue testing that is currently used for total knee replacement tibial trays. Once the standard has been approved it will be used by medical device companies as well as testing laboratories. The purpose of the test will be to indicate that the tibial metal component will not fail over its expected lifetime.
All interested parties are invited to join in the ongoing development of WK45235
CONTACT Technical Information: Gokce Yildirim, Stryker MAKO • Jersey City, N.J. • Phone: 973-946-6994 | ASTM Staff: Pat Picariello • Phone: 610-832-9720 | Upcoming Meeting: May 6-9, 2014 • May Committee Week • Toronto, Ontario, Canada
 SN Home
SN Home Archive
Archive Advertisers
Advertisers Masthead
Masthead RateCard
RateCard Subscribe
Subscribe Email Editor
Email Editor