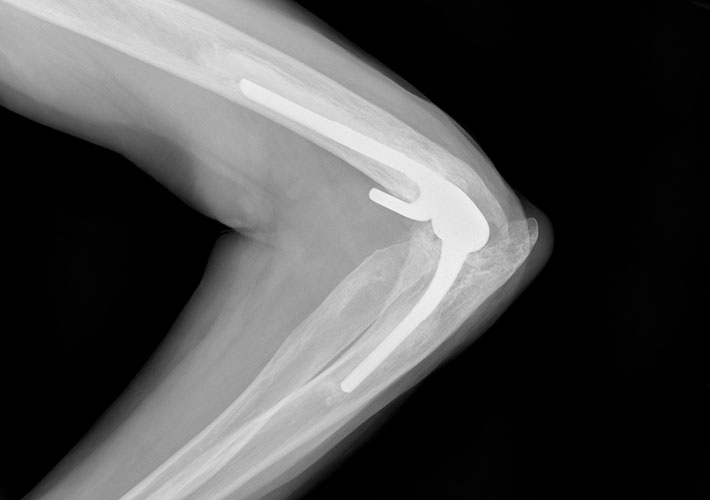
Elbow Prostheses
ASTM International’s committee on medical and surgical materials and devices (F04) has updated its standard on elbow prostheses to include testing guidance. This is the first time that any standards organization in the world has incorporated testing into a specification for these products, according to the committee.
The revision to the specification for total elbow prostheses (F2887) will help manufacturers, regulatory bodies, and test laboratories, according to Brian Kincaid, an ASTM International member. These groups will benefit from the testing guidance to help objectively evaluate the safety and efficacy of total elbow replacement (TER) devices under laboratory conditions that are commonplace for other large total joints.
“These updates provide references to peer-reviewed literature that describes bench-top testing methodologies in detail, and address the many performance requirements in F2887,” says Kincaid, a manager at Zimmer Biomet. “Prior to this revision, the specification had no specific testing guidance. These methods in totality represent the first such testing standards for TER devices worldwide.”
According to Kincaid, this update will help foster benchmarking of safety and efficacy for new TER designs, with the goal of better clinical outcomes and higher patient satisfaction.
The methods described in the standard are specific to the “linked semi-constrained” style of TER. Kincaid says the committee welcomes anyone interested in helping integrate methods for other designs such as hemi and radial head.
 SN Home
SN Home Archive
Archive Advertisers
Advertisers Masthead
Masthead RateCard
RateCard Subscribe
Subscribe Email Editor
Email Editor