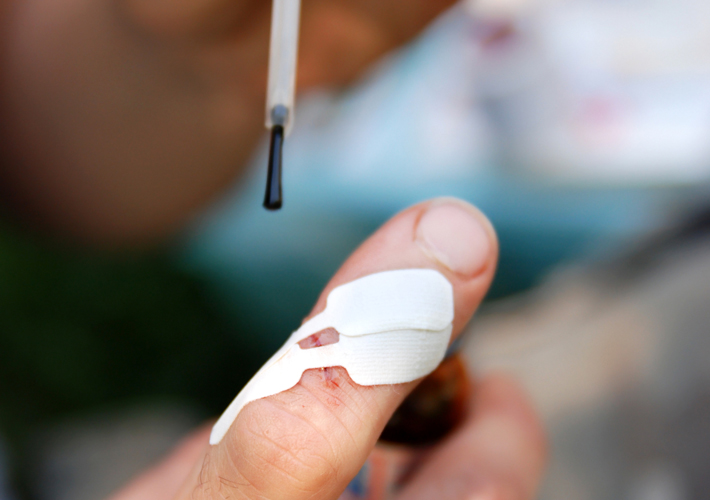
Microbial Barrier Properties of Wound Dressings
ASTM International’s committee on pesticides, antimicrobials, and alternative control agents (E35) is developing a proposed standard that will provide guidelines on how to test microbial barrier properties of solid wound dressings that include liquid adhesives.
Solid wound dressings include those with or without antimicrobial agents in which the manufacturer is claiming microbial barrier properties in their performance claim. The barrier can be a physical (polyurethane) barrier or an antimicrobial barrier.
Liquid adhesives also protect the wound from being infected from microorganisms. Liquid adhesive dressings contain cyanoacrylate or the polyacrylate, which polymerize quickly to create a solid barrier and protect the wound from contamination.
ASTM International member Shazia Siddiqui notes that while several published standards assess the efficacy of solid wound dressing, none provide instructions to demonstrate the barrier properties of solid wound dressing that include liquid adhesives. The proposed standard (WK81255) will fill that need.
Siddiqui says that all interested parties are invited to participate in the ongoing development of the proposed standard. The task group is particularly interested in participants who will review the standard and advise if more information or clarification is needed.
ASTM welcomes participation in the development of its standards. JOIN ASTM.
 SN Home
SN Home Archive
Archive Advertisers
Advertisers Masthead
Masthead RateCard
RateCard Subscribe
Subscribe Email Editor
Email Editor