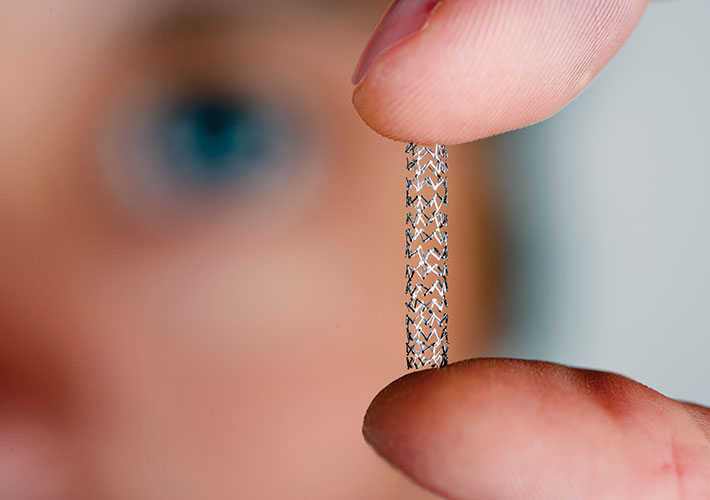
New Standard Supports High-Strength Alloy for Stents, Other Surgical Implants
A new ASTM International standard covers a high- strength alloy that could be used for cardiovascular stents as well as trauma, spinal, and face-related surgical implants.
“This powder-metallurgy alloy has a good combination of high strength and high ductility when compared to other metallic implant materials,” said ASTM International member John Disegi, a consultant at Advanced Biomaterial Consulting LLC. He noted that this could allow product engineers to “downsize” various existing implants.
Technically speaking, the standard is a specification for the chemical, mechanical, and metallurgical requirements for “wrought molybdenum-47.5 rhenium alloy.” The specification (known as F3273) was created by ASTM International’s committee on medical and surgical materials and devices (F04).
The material covered in the new standard could be of interest to both regulatory bodies and consumers for its biocompatibility. Because the alloy does not contain elements such as nickel, chromium, or cobalt, it is less likely to cause metal allergy reactions, according to Disegi.
The new standard will be highlighted at the next North American Spine Society meeting, October 25-28, in Orlando, Fla.
Read more about how standards support health and medicine in “Standards Advance Patient Care." Also see this overview: Standards for Healthcare Services, Products, and Technology.
 SN Home
SN Home Archive
Archive Advertisers
Advertisers Masthead
Masthead RateCard
RateCard Subscribe
Subscribe Email Editor
Email Editor